ISSUE1713
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Review the efficacy and safety of mResvia, an mRNA respiratory syncytial virus (RSV) vaccine, for prevention of lower respiratory tract disease caused by RSV in adults ≥60 years old.
- Description: An mRNA respiratory syncytial virus (RSV) vaccine encoding a stabilized prefusion form of the RSV fusion (F) glycoprotein.
- Indication: FDA-approved for prevention of lower respiratory tract disease (LRTD) caused by RSV in adults ≥60 years old.
- Efficacy: A single dose has prevented RSV LRTD in adults ≥60 years old compared to placebo. One dose of mResvia appears to be less effective over two RSV seasons than one dose of a recombinant RSV vaccine (Arexvy or Abrysvo).
- Adverse Effects: Most common are injection-site pain, axillary swelling or tenderness, fatigue, headache, myalgia, arthralgia, and chills.
- Dosage: A single 50 mcg/0.5 mL IM dose.
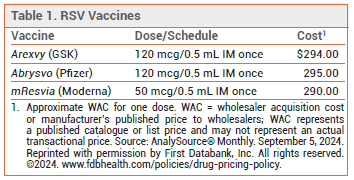
- Cost: The wholesale acquisition cost for one dose is $290.
- Conclusion: The CDC Advisory Committee on Immunization Practices (ACIP) recommends a single dose of any available RSV vaccine for all adults ≥75 years old and for those 60-74 years old at increased risk of severe RSV disease.
Outline
Tables |
The FDA has licensed mResvia (Moderna), an mRNA respiratory syncytial virus (RSV) vaccine, for prevention of lower respiratory tract disease (LRTD) caused by RSV in adults ≥60 years old. It is the first mRNA vaccine to be licensed in the US for this indication. Two recombinant RSV vaccines, Arexvy and Abrysvo, are also available for prevention of RSV LRTD.1 Arexvy is approved for use in adults ≥50 years old.2 Abrysvo is approved for use in adults ≥60 years old and in pregnant women to prevent RSV LRTD in their infants.
RSV DISEASE — RSV typically causes a mild upper respiratory tract infection in adults, but older adults, particularly those with underlying health conditions, have an increased risk of RSV-associated hospitalization. RSV epidemics in the Northern Hemisphere typically occur between October and April, peaking in December or January.
EFFICACY OF RECOMBINANT VACCINES — Both Arexvy and Abrysvo have been shown to reduce the incidence of RSV LRTD in adults ≥60 years old.1 A case-control analysis of patients ≥60 years old hospitalized for acute respiratory illness found that vaccination with a recombinant RSV vaccine was about 75% effective against RSV-associated hospitalization during the first season of use.3 Use of Abrysvo in pregnant women reduced the incidence of medically-attended RSV LRTD in their infants during one RSV season.1
THE NEW VACCINE — mResvia is a lipid nanoparticle-encapsulated, mRNA-based vaccine. It encodes a stabilized prefusion form of the RSV fusion (F) glycoprotein derived from an RSV A strain. The RSV prefusion F glycoprotein mediates viral fusion and host-cell entry and elicits neutralizing antibodies. The same antigen is used in Arexvy and Abrysvo.
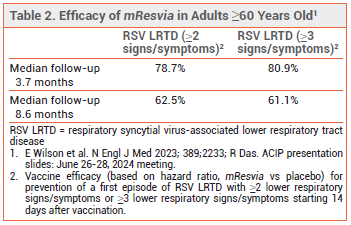
CLINICAL STUDIES — FDA approval of mResvia was based on the preliminary results of an ongoing observer-blinded trial (ConquerRSV) in 35,064 adults ≥60 years old who were randomized to receive a single dose of mResvia or placebo. The vaccine was effective, compared to placebo, in preventing a first episode of RSV LRTD (see Table 2). Vaccine efficacy against RSV-associated acute respiratory disease was 68.4%; efficacy against RSV A was higher than that against RSV B (78.5% vs 51.7%).4
A follow-up report found that a single dose of mResvia was ~50% effective in preventing RSV LRTD over 18 months in adults ≥60 years old.5 Higher efficacy rates against RSV LRTD over two RSV seasons have been reported following a single dose of Arexvy or Abrysvo (67.7% over 23.3 months with Arexvy and 81.5% over 16.4 months with Abrysvo).6,7
ADVERSE EFFECTS — In the ConquerRSV trial, the most common local adverse effects reported within 7 days of mResvia vaccination were injection-site pain (55.9%) and axillary swelling or tenderness (15.2%). Systemic adverse effects included fatigue (30.8%), headache (26.7%), myalgia (25.6%), arthralgia (21.7%), and chills (11.6%). More cases of urticaria were reported with mResvia than with placebo within 28 days after administration (15 vs 5). One participant in the mResvia group developed facial paralysis 4 days after vaccination. Atrial fibrillation and Guillain-Barré syndrome (GBS) were reported following administration of Arexvy or Abrysvo in clinical trials. Vaccine-related cardiac arrhythmias and GBS were not reported in clinical trials with mResvia.5
ACIP RECOMMENDATIONS — The CDC Advisory Committee on Immunization Practices (ACIP) has updated its recommendations for use of RSV vaccines in older adults. A single dose of an RSV vaccine is now recommended for all adults ≥75 years old and for those 60-74 years old at increased risk of severe RSV disease (see Table 3).8 For optimal protection, the vaccine should be given before the onset of the RSV season. Administration of an RSV vaccine and other adult vaccines during the same visit is considered acceptable, but local or systemic reactogenicity could increase.9
DOSAGE AND ADMINISTRATION — The mResvia vaccine is supplied as a frozen suspension in prefilled syringes containing a single 50 mcg/0.5 mL IM dose. The syringe can be thawed in the refrigerator for 60 minutes and should stand at room temperature for 10-20 minutes before administration. A syringe that is thawed at room temperature is ready to be administered after 45 minutes.
CONCLUSION — A single dose of mResvia, the first mRNA respiratory syncytial virus (RSV) vaccine, was effective in preventing RSV-associated lower respiratory tract disease during one RSV season in adults ≥60 years old. Follow-up data suggest that one dose of mResvia may offer less protection over two RSV seasons than one dose of Arexvy or Abrysvo, the recombinant RSV vaccines available in the US. The CDC Advisory Committee on Immunization Practices recommends a single dose of any available RSV vaccine for all adults ≥75 years old and for those 60-74 years old at increased risk of severe RSV disease.
- Two vaccines (Arexvy and Abrysvo) for prevention of RSV disease. Med Lett Drugs Ther 2023; 65:155.
- In brief: RSV vaccine (Arexvy) for ages 50-59. Med Lett Drugs Ther 2024; 66:113.
- D Surie et al. RSV vaccine effectiveness against hospitalization among US adults 60 years and older. JAMA 2024 Sep 4 (epub). doi:10.1001/jama.2024.15775
- E Wilson et al. Efficacy and safety of an mRNA-based RSV preF vaccine in older adults. N Engl J Med 2023; 389:2233. doi:10.1056/nejmoa2307079
- R Das. Update on Moderna's RSV vaccine, mResvia (mRNA-1345), in adults ≥60 years of age. ACIP presentation slides: June 26-28, 2024 meeting. Available at: https://bit. ly/4ciO7hH. Accessed September 26, 2024.
- I Munjal. RSVpreF adult clinical development update. ACIP presentation slides: June 26-28, 2024 meeting. Available at: https://bit.ly/4ciO7hH. Accessed September 26, 2024.
- S Gerber. Arexvy (adjuvanted RSVPreF3) 2-year update. ACIP presentation slides: June 26-28, 2024 meeting. Available at: https://bit.ly/4ciO7hH. Accessed September 26, 2024.
- A Britton et al. Use of respiratory syncytial virus vaccines in adults aged ≥60 years: updated recommendations of the Advisory Committee on Immunization Practices – United States, 2024. MMWR Morb Mortal Wkly Rep 2024; 73:696. doi:10.15585/mmwr.mm7332e1
- CDC. Vaccines and immunizations. Healthcare providers: RSV vaccination for adults 60 years of age and over. July 3, 2024. Available at: https://bit.ly/3yC0A1n. Accessed September 26, 2024.